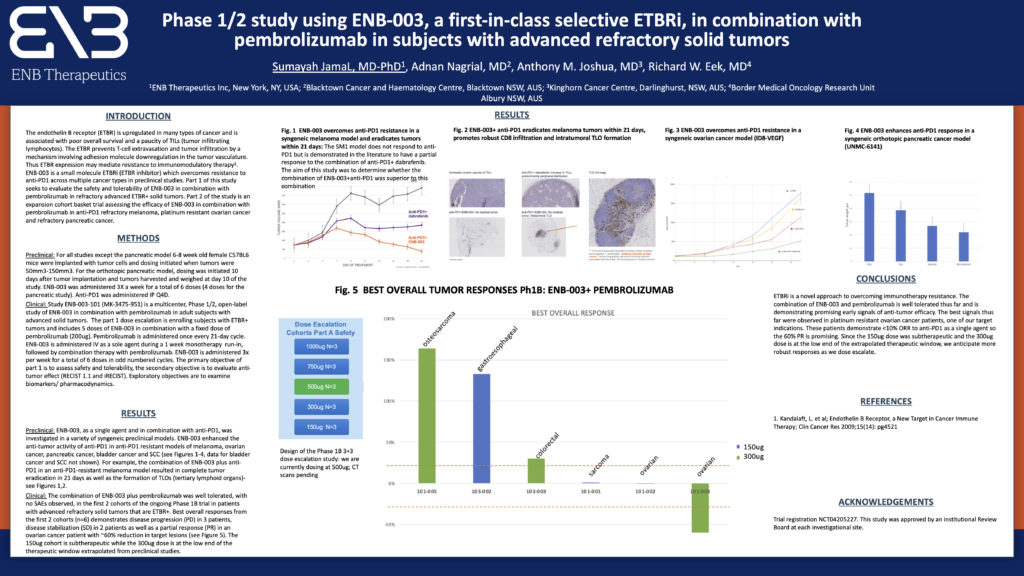
ENB Therapeutics reports preliminary data from the ongoing Phase 1/2 trial assessing the safety and efficacy of lead product ENB-003 in combination with pembrolizumab. Safety data are encouraging with no significant drug related safety events from the first 3 dosing cohorts. Best overall responses from the first 6 patients include 2 disease stabilizations and 1 confirmed partial response. The most encouraging responses were observed in 2 patients with platinum-resistant ovarian cancer who demonstrated 6% and 60% reduction in target lesions at the 150ug and 300ug doses respectively. The 150ug dose was sub therapeutic based upon weight and we anticipate enhanced efficacy as we continue to dose escalate.
Download the poster by clicking the link below.